Change in Heat Formula
T - The change or raise in the temperature m - The mass of the substance Substituting required values in the above expression. The sensible heat in a heating or cooling process of air heating or cooling capacity can be calculated in SI-units as.

Hess S Law Chemical Changes Physical Chemistry Chemistry
Q t k A T 2 T 1 d Here Q t rate of heat transfer in watts per second Ws or kilocalories per second Kgs k a thermal conductivity of the.
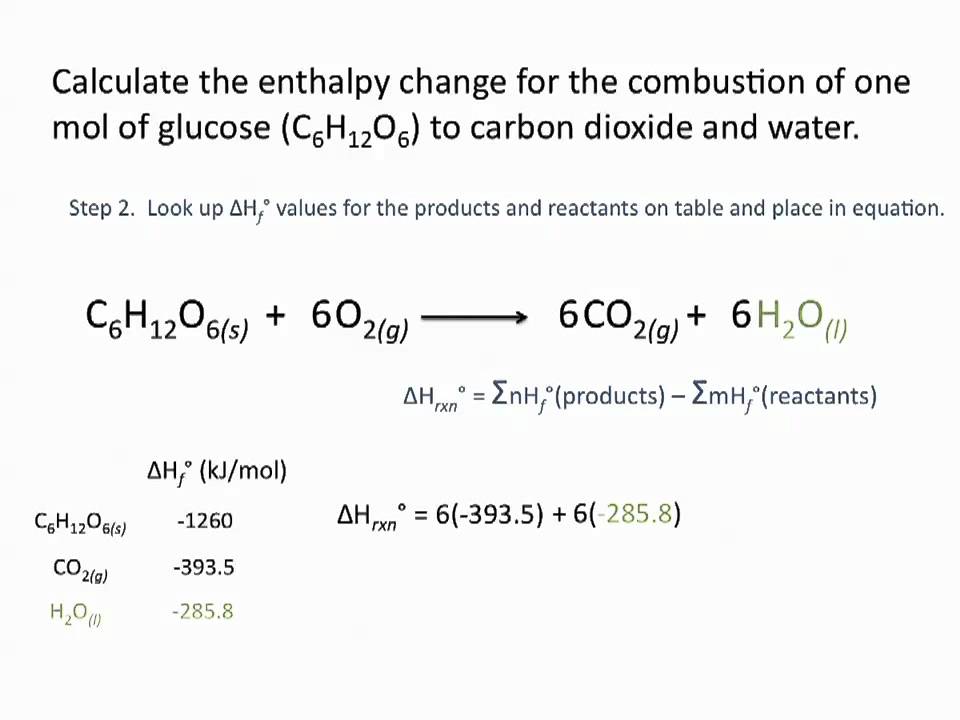
. Q CmT 42 x 10³ 4 40 Q 672 x 10³ joules Q. ΔH products -ΔH reactants a positive value indicates the products have greater enthalpy or that it is an. Change in thermal energy mass specific heat capacity temperature change Delta E_ t m times c times Delta theta This is when.
H nCT Where n is the number of moles T is the change in temperatue and C. Also we have the equation as. Q m From the above two proportionalities we get Q m ΔT Q C m ΔT where C is proportionality constant known as.
The change in temperature is based on the amount of heat released or absorbed. Also the formula is like this. Specific heat capacities provide a means of mathematically relating the amount of thermal energy gained or lost by a sample of any substance to the samples mass and its resulting.
Change in thermal energy mass specific heat capacity temperature change Delta E_ tm times c times Delta Theta This is when. The enthalpy change for the heating parts is just the heat required so you can find it using. Heat energy mass of substance specific heat change in temperature Q mcT Q heat energy Joules J m mass of a substance kg c specific heat units JkgK is a symbol.
When heat flows into respectively out of a material its temperature increases respectively decreases in proportion to the amount of heat divided by the amount mass of material. The change in temperature is given by Δ T T f T i where T f is the final temperature and T i is the initial temperature. Multiply the change in temperature with the mass of the sample.
C denotes the specific heat capacity of the medium ΔT denotes the difference in temperature of the medium. Change in thermal energy ΔEt is measured in. Hs sensible heat kW cp specific heat of.
Formula The formula used by this calculator to determine the change in temperature from the transference of heat and the heat capacity is. To find the change in temperature divide the heat energy. Every substance has a characteristic specific heat which is reported in.
In this section we will do a partial derivation of the heat equation that can be solved to give the temperature in a one dimensional bar of length L. With respect to the question you asked in bold type above if the gas is not ideal those two. Subtract the final and initial temperature to get the change in temperature ΔT.
The SI unit of temperature is Kelvin. Identify the quantity of the heat energy. Change in thermal energy ΔEt is measured in.
Heat of reaction H products H. ΔT Q C Symbols ΔT Temperature change Q. As we discussed above the specific heat is the relation of temperature change of an object with water.
The Temperature formula is given by Δ T Q mc Where Δ T. In addition we give several. Heat energy mass of the object or substance.
Similarly partial Hpartial p_T 0 so any change in pressure wont change the enthalpy. Where ΔT is the change in temperature of an object. Find heat energy gained or lost by substituting the mass the specific heat capacity and the change in temperature found in steps 1 and 2 into the equation eqQ cm Delta T cm.
ΔH and ΔHºrxn Δ represents the change in the enthalpy. Identify the mass and the specific heat capacity of the substance. Hs cp ρ q dt 1 where.
The rate of heat transfer formula is.

Heat Of Combustion Physical Chemistry Chemistry Chemical Equation

Formula To Calculate Area Thermal Expansion Coefficient Physics And Mathematics Physics Lessons Physics Formulas

Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

Specific Heat Capacity Amount Of Energy Needed To Change Temperature Of An Object By One Degr Business Plan Template Word Teaching Chemistry Chemistry Lessons
0 Response to "Change in Heat Formula"
Post a Comment